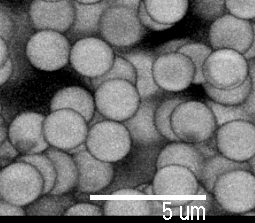
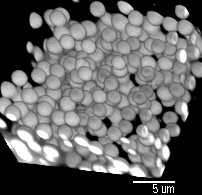
Pictures by Piotr Habdas, showing colloidal particles which have formed a gel. Another image of the same sample is the background for this web page.
We can do lots of cool things. Our current capabilities (August 2025) include:
|
| Magnification/NA | theoretical resolution | working distance | comments |
|---|---|---|---|
| 1.6x / 0.05 air | 6 um | 3.4 mm | - |
| 5x / 0.15 air | 3.3 um | 12 mm | - |
| 10x / 0.22 air | 1.3 um | 5.8 mm | - |
| 10x / 0.40 air | 0.7 um | 2.2 mm | - |
| 10x / 0.40 oil | 0.7 um | 0.36 mm | - |
| 20x / 0.40 air | 0.7 um | 1.9 - 3.2 mm | long working distance lens, with a correction collar for glass thicknesses from 0.0 to 2.0 mm |
| 20x / 0.70 multi (oil) | 0.4 um | 250 um | This lens can work with a variety of immersion fluids, including water, glycerol, and oil. In general, consider this an oil immersion lens. |
| 40x / 0.55 air | 0.5 um | 1.9 - 3.3 mm | long working distance lens, with a correction collar for glass thicknesses from 0.0 to 2.0 mm |
| 40x / 1.25 oil | 0.23 um | 100 um | - |
| 63x / 0.70 air | 0.4 um | 1.8 mm | long working distance lens, with a correction collar for glass thicknesses from 0.1 mm thick up to 1.3 mm (in other words, a microscope slide or petri dish). |
| 63x / 1.20 water | 0.24 um | 220 um | Best lens for aqueous samples, such as most biological samples. This is a really nice lens. |
| 100x / 1.35 oil | 0.21 um | 90 um | - |
| 100x / 1.40 oil | 0.20 um | 90 um | The "highest power" lens. It helps if your samples have an index of refraction similar to oil, in other words, close to n=1.5. |
NOTES:


Pictures by
Piotr
Habdas, showing colloidal particles
which have formed a gel. Another image of the same sample is
the background for this web page.
![]()