Emulsions
2D Emulsions
We say a substance is an emulsions when at least one liquid is dispersed into another immiscible liquid. Of course this definition doesn't make much sense. How can two immiscible liquids mix? For example, oil and water will separate when mixed. The simplest answer is that two immiscible liquids can't be mixed without something being added to make them mix. This something is called a surfactant.
If a common suffactant such as SDS (sodium dodecyl sulfate) is added to an oil water mixture and shaken, little stable oil droplets will form within the water. The reason oil droplets can form without separating into one liquid mass is because the surfacant "stabalizes" the oil-water interface. Normally oil and water don't like each other, meaning they prefer not to be in contact (or share an interface). When no surfactant is added the two liquids minimize the interface they share by seperating into two phases with oil on top and water on bottom. Surfactant on the other hand would prefer to be in water and oil at the same time. So the following happens when water, oil, and SDS is shaken together. First the shaking breaks the oil up into little droplets, and then the SDS, liking both water and oil, attaches to the surface of the oil droplets. With SDS at the surface of all the oil droplets, this prevents two touching droplets from coalescing (or recombining) into one big oil droplet, forming a stable emulsion.
The image below illustrates the concepts just mentioned. The blue green area is the water, the yellow area is an oil droplet, and blue ojects with red tails are the surfactant molecules which attach to the surface of the oil droplet. I've taken this picture from Eric Weeks' cite.
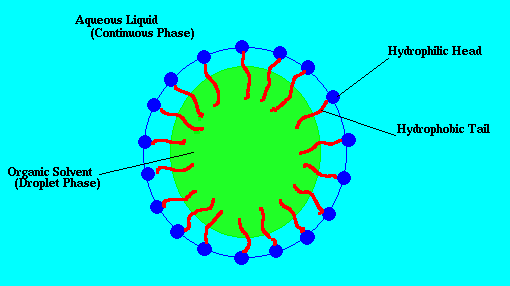
Emulsions are very common and encountered everyday in the kitchen from the foods you eat to the soapy water used to clean your dishes. Many foods such as mayonaise, ice cream, and egg yolk are emulsions. The taste of these edile emulsions can be finely tuned by adjusting the properties of the surfactant used and the size of the emulsion droplets. After eating your edible emulsions the dishes must somehow be cleaned. An effective choice is soap because it contains surfactant. When wash your greasy hands or dishes with soap you are actually making an emulsion. The surfactants in the soap help to remove the oils from your hands and form droplets in the water. Without the surfactant the oil would not want to mix with the water, and your hands would remain greasy. Because of the wide uses of emulsions they are of great interest to many industries, and are studied by many different fields of science and engineering.
| 